Did you know that not all LVDS platelets are the same?
Vitalant provides platelets with the longest potential shelf life available to hospitals without secondary testing.
While all large-volume delayed sampling (LVDS) platelets are considered ‘therapeutically equivalent’ with all strategies listed in the FDA Guidance, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” shelf life for LVDS can vary based on the mitigation strategy selected by the blood center.
Did You Know?
Vitalant is providing platelets with the longest potential shelf life available to the hospital without secondary testing . While all LVDS platelets are considered “therapeutically equivalent” with all strategies listed in the FDA Guidance, “Bacterial Risk Control Strategies for Blood Collection Establishments and Transfusion Services to Enhance the Safety and Availability of Platelets for Transfusion,” shelf life for LVDS can vary based on the mitigation strategy selected by the blood center.
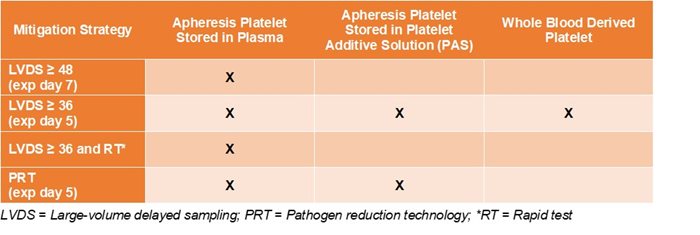
For LVDS ≥ 36, hospitals may be able to extend the shelf life for day 6 and day 7 using rapid bacterial testing only if the blood center collects apheresis platelets stored in plasma and has a storage container cleared or approved by FDA for 7-day storage. If the hospital chooses to do this, please consider:
- The blood center or transfusion service that performs the secondary testing must update the container label to reflect the new expiration date as required by 21 CFR 606.121(c)(4)(i).
- Extending expiration beyond 5 days is a manufacturing procedure requiring FDA registration and blood product listing, as defined in 21 CFR 607.3(d).
Vitalant Offerings
Vitalant evaluated all options listed in the Final Guidance with three key considerations in mind:
- Provide a safe and sufficient supply of platelets
- Provide cost-effective options for our hospital customers
- Limit total number of options to improve inventory management and facilitate ease of use
Vitalant selected LVDS 48-hour/7-day and Pathogen Reduction Technology (PRT), also known as psoralen-treated platelets, to meet the key considerations listed above, to continue to provide organizations clinical choices and to leverage our national footprint. We have prioritized transfusion-ready options that do not require additional testing.
Source: AABB, Association for the Advancement of Blood & Biotherapies website https://www.aabb.org/docs/default-source/default-document-library/resources/association-bulletins/ab21-02.pdf?sfvrsn=6c304e60_0
